Wednesday 25 December 2013
Thursday 7 November 2013
勇気100%
垂頭喪氣 低聲哭泣 你怎麼啦
有著陽光般燦爛笑容的你到哪兒去啦
喔喔 想做的事情 做了再說 這才是青春
任何難過的時候 我都在你身邊
夢想如果不宏大的話 那多無趣呀
就讓我們拍拍胸脯去冒險囉
嘿嘿!
對呀 用100%的勇氣 能做的就是更努力嘛
緊抱著這個世界上的元氣
對呀 用100%的勇氣 能做的就是更盡力嘛
我們所擁有的光輝 請永遠不要忘記呦
又是跌撞 又是創傷 做的話還是比較好嘛
如果心中的熱情正燃燒著 就千萬別後悔
喔喔 原地不動的話 就無法啟動 心中的那份悸動
我喜歡和你去追趕的風呦
如果昨天沒能翱翔的天空還存在的話
那就讓我們試著抓住現在已有的機會吧
嘿嘿!
對呀 用100%的勇氣 能做的就是更投入嘛
現在還不是只靠流淚就能結束的時候吧
對呀 用100%的勇氣 已經無法再回頭了
我們要以我們的風采去迎向任一個地方呀
儘管超級寂寞的夜晚已降臨
但嶄新的早晨也必將來到嘛
對呀 用100%的勇氣 能做的就是更努力嘛
緊抱著這個世界上的元氣
對呀 用100%的勇氣 能做的就是更盡力嘛
我們所擁有的光輝 請永遠不要忘記呦
嘿嘿嘿!
有著陽光般燦爛笑容的你到哪兒去啦
喔喔 想做的事情 做了再說 這才是青春
任何難過的時候 我都在你身邊
夢想如果不宏大的話 那多無趣呀
就讓我們拍拍胸脯去冒險囉
嘿嘿!
對呀 用100%的勇氣 能做的就是更努力嘛
緊抱著這個世界上的元氣
對呀 用100%的勇氣 能做的就是更盡力嘛
我們所擁有的光輝 請永遠不要忘記呦
又是跌撞 又是創傷 做的話還是比較好嘛
如果心中的熱情正燃燒著 就千萬別後悔
喔喔 原地不動的話 就無法啟動 心中的那份悸動
我喜歡和你去追趕的風呦
如果昨天沒能翱翔的天空還存在的話
那就讓我們試著抓住現在已有的機會吧
嘿嘿!
對呀 用100%的勇氣 能做的就是更投入嘛
現在還不是只靠流淚就能結束的時候吧
對呀 用100%的勇氣 已經無法再回頭了
我們要以我們的風采去迎向任一個地方呀
儘管超級寂寞的夜晚已降臨
但嶄新的早晨也必將來到嘛
對呀 用100%的勇氣 能做的就是更努力嘛
緊抱著這個世界上的元氣
對呀 用100%的勇氣 能做的就是更盡力嘛
我們所擁有的光輝 請永遠不要忘記呦
嘿嘿嘿!
Tuesday 1 October 2013
A tribute to doctors
https://docs.google.com/document/d/1WlgMPvEdrmZuCOvSxIbNmktDRRy9kz_aGzI03sQdIh4/preview
《醫生》
醫生是當今社會最受歡迎的行業。論其工作意義,社會地位,薪酬前途,挑戰性和成就感等各方面,都十分令人滿意。根據兒童圖書《12歲決定一生》的研究調查顯示,一千個12歲台灣小學生的志願之中,排行第一位的職業就是醫生,其人數遠超過第二位的老師足足一倍。在大學芸芸眾多學科之中,醫學院的收生成績往往名列前三甲,即使優先取錄計劃的拔尖生,分分鐘亦未必能夠入讀醫學院。可見醫生這個行業的江湖地位,已經深深植根在新一代孩童以及年青人的夢想之中。
為甚麼這麼多孩子想做醫生呢?當然是因為醫生擔當拯救生命的工作,是人類健康的守護天使,而世間上沒有任何事物比生命更重要的了,因此醫生幾乎是世界上最有意義的工作。沒錯,當我還是小孩子的時候,我也有這麼一種想法。時至今日,我依然覺得醫生是個偉大和甚有意義的職業,我對醫生也十分尊敬和欽佩。不過,我卻不認為「偉大」或「有意義」是這麼多人想做醫生,以及醫學院招生成績這麼高的原因。醫生這項職業這麼受孩子歡迎,每一個孩子都想當個濟世為懷的醫師,完全是上一代教育貫輸的成果。單單是父母期望這個重要因素,就足以醫生的江湖地位傳承下去。
其實這個世界上聽起來有意義的職業很多,單單是拯救生命有關的工作,就不下數十種。護士、助產士、消防員、救生員、飛行服務隊甚麼的,通通都是把別人的生命懸於股掌中的職業,但他們的社會地位和待遇遠遠不如醫生。像牙醫這樣的專科醫生,每天幹著幫人家洗牙剝牙的活,左看右看也跟所謂拯救生命的職稱距離甚遠。但他們的社會待遇,卻不是救人於水深火熱中的救生員,每小時三十幾元的人工所能比擬的。同樣是拯救生命的職業,相比之下,醫生不見得特別偉大,只是工作的難度相對較高,所需的專業知識較淵博,因此才能有效地壟斷巿場,佔據較高的社會地位。
從醫的難處
在現今社會,即使不至於大力游說,幾乎沒有一個父母會反對自己的孩子當醫生的。原因很簡單:當醫生薪酬高,福利好,屬於專業人士,整個行業的社會地位牢不可破,其工作備受社會各界尊重。只是,大家都鮮少提到醫生這行業的苦處,一般孩子推卻父母的原因都總是沒有興趣和怕見血罷了。就我這個外行人毫無說服力的觀察,這個行業的主要難處有三,就是門鑑高、工時長和壓力大。門鑑高是人所共知的第一重關卡,首先考進醫學院已經需要極之優異的成績,在讀醫的期間也要反覆背誦無數的內容。而且,醫科生所需的專業培訓特別漫長,讀醫所需的時間和金錢成本也比其他學科要多(香港本科五年制的課程已經算短了,外國不少大學要求入讀醫學院前,必須先完成學士課程,導致醫科生們甫一畢業已經三十歲了)。這些高成本的專業培訓也直接導致另外兩個做醫生的苦處:工時長和壓力大。
一般公立醫院實習醫生和初入職的正式醫生,一星期工作六天,每天一般的工作時間為十小時,可是每星期約莫有一至兩夜通宵on call。所謂通宵on call,也就是首先日間從上午八時工作至晚上,然後一整夜通宵繼續開工,第二朝天亮了,卻不可以回家休息,繼續第二天上午八時至下午六時的工作。換言之,必須連續工作三十四小時,這樣的生活一星期要過一至兩次。其苦況不單止朝九晚五的辦公室工作不能與之相比,早午晚輪更的護士不能相提並論,連出名虐待員工的四大會計師樓,也不至於連續工作三十幾個小時。個人認為,這種不人道的工時嚴重摧殘一個人的意志,磨滅一個醫生濟世為懷的熱誠,不單止剝奪了日常生活和興趣,連醫生本人的健康也剝削了,真是諷刺得很。這種極端疲勞的狀況下,加上醫院充斥著生離死別的慘淡氣氛,諸般壓力煎熬下,不發生醫療失誤事故已經是奇跡,要長期保持愉快的心情工作幾乎絕不可能。
之所於要把新入職的醫生虐待至如斯地步,很明顯是為了最大化經濟成本效益的緣故。培訓一個醫生,花了這麼多人力物力,當然要在他有能力脫離建制之前(也就是開診所自立門戶)物盡其用。越是矜貴的專業人士,初入行時越容易被公司或政府抽光所有的時間生命,這不單止是醫生,在會計師和律師等行業也有這種情況。畢竟專業人士的培訓成本太貴了,這個社會寧願妄顧這些人的感受,也不願多花資源培訓多一點人才分擔他們的工作。相對而言,同在醫院工作,護士就沒有這樣要命的工時。因為護士所需的醫學知識沒有醫生般深入和詳細,其工資和培訓成本較低,跟醫生之間有著明顯的階級分野,所以每年招聘的人數可以遠多於醫生。因此,醫生和護士這對宿命的配搭,一個相對人工低、還得聽人呼喝指使,一個相對工時長、卻得肩負最大的責任,各有各的苦況,不能理解兼沒有共鳴,難怪醫生和護士永遠也是天敵,彼此怨恨爭吵不休。
從醫的社會地位
這個社會之所以對醫生如此要求,把醫生逼至為了工作廢寢忘餐的地步,完全是因為醫生的社會地位高崇,因此必須付出更多以回應社會的期望。究其原因,也是因為大家對醫生有一種難以忘捨的情意結,以致把醫生的地位大力抬高,甚至遠超過其本來應該身處的位份。
醫生是一個歷史悠久的職業,不論中國抑或西方,幾千年前就已經有醫生(大夫)的存在。可是,古時候中國的大夫,社會地位一點也不高。像扁鵲和華佗等名醫,之所以名得以經傳,也是因為他們在醫學造詣上有創新的研發(學者的職份),而不單止是因為他們醫好了多少個病人(醫生的職份)。北宋名相范仲淹小時候由於樂於助人,曾立志當一名大夫,希望醫好有需要的病人,身邊的朋友都覺得莫名奇妙,因為大夫並不見得是甚麼理想職業,大伙兒反而支持他考取功名在朝廷當宰相。後來,醫生這個職業逐漸變成越來越備受看重,我估計主要是因為病菌在人類社會中越來越放肆,為了保命,人類對現代醫學的需求日增,醫生作為人類與病菌之戰的前鋒,其重要性才越來越得到肯定。
根據《槍炮、病菌、鋼鐵》一書所述,現今最普遍的群聚疾病(例如流行性感冒、霍亂、痳疹等)是從人口密集的人類社群中才得以發展起來。遠古時代的狩獵採集部落,人口數量不足以讓群聚疾病長期繁衍和演化。要是一個只有數百人口的部落得了傳染病,病毒一下子感染全體人口,生病的人要嘛病死,要嘛康復之後產生抗體,於是沒有擴散空間的病毒必定在短期內絕跡。託近代如雨後春昏般湧現的城巿的洪福,群聚疾病的病原體在密集的人類社群有充裕的時間發展演化。像公元一世紀發生在羅馬的安東尼瘟疫,公元五四二年出現在歐洲的腺鼠疫,以及一三四六年名滿天下的黑死病,都集中在人口密集的城巿肆虐。
因此,醫生的角色在封閉的農村,或許功效不致於太顯著,但在隨時大規模爆發疫症的大城巿,醫生就是保家衛國的戰士,理所當然備受尊崇了。古時候,各地城巿化的步伐遠不如今日之迅速,城巿與城巿之間的交流也遠不及今日之頻繁,故此把關病菌入侵大門的職責也不似今日般艱巨困難。更重要的是,古時的醫學技術發展不足以應對大規模擴散的群聚疾病,瘟疫蔓延就有如死神到訪,醫生們也束手無策。但是最近二百年醫學的進步,為醫生們提供大量跟疾病作戰的有效兵器。有了武器的支援,醫生們工作的成效比從前顯著多了,成了捍衛人類的常勝軍,越來越備受尊重也是理所當然的事。醫學的迅速發展,連帶一整個行業都冒起頭來,管您是牙醫還是獸醫,只要掛著醫生的名號,就是拯救生命的大軍之一員,能跟一眾救急扶危的醫生共享尊貴的榮譽。
從醫生的實質意義
以上數段嘗試約略從歷史角度,看醫生這行業的演化進程。可是,真正的醫生才不管這些。真正的醫生之所以能在嚴苛的工時和工作環境底下支持下去,除了優厚的薪酬和地位之外,不多不少,也基於心裡認同自己做的,是十分偉大和有意義的工作。
於是,我們不期然想到一個問題:救人一命是不是真的如此偉大?是不是真的如此有意義呢?別忘了,本來人人總有一死,只是遲早的問題。一個醫生花心機時間救活一個病人,並不代表他將來不會死,只是稍為延長其壽命罷了。可是生命若有定數,要躲的還總是躲不過。求生雖然是人的本能,但對於有些人來說,死得太早,還不及死得太晚更來得坎坷。在自殺率節節上升的當代社會,執著於「救人一命勝過七級浮屠」,說不定只是行醫者的一廂情願罷了。
即使不論這些特殊例子,單從醫病這個角度看醫生的職責,我們能夠斷言醫生的工作,比其他各行各業來得更偉大?更有意義嗎?畢竟一項工作的意義,不是一個永恒的標準,而是由從事的人自己賦予的。歷史上有很多有遠大抱負的大人物,都對醫學有一定的接觸,卻因為要救更多的人,放棄當醫生的打算。魯迅棄醫從文、孫中山熱心於革命,這些都是家傳戶喻的故事。
一個醫生,窮一生努力治病,頂多能救治幾千幾萬人。只是放眼世界,且不論一場戰爭,每每死傷幾百萬人不等,單是根據慈善團體的資料。非洲每7秒鐘就有一位孩子因飢餓或疾病而死去。換言之,非洲一天之內失救而死的兒童數目,甚至多於一個普通醫生窮一生所醫治的病人。談到人命的價值寶貴,大家還是寧願買樓買車也不願意掏腰包捐錢。畢竟非洲的人命不值錢嘛,香港的人命相對矜貴多了。同樣的醫療成本,要醫的話也先醫高度文明的香港人,這種才符合最大經濟效益。偉大的醫生在香港註冊行醫,延長香港人的壽命,自然對社會文明的前進作出偉大的貢獻了。至於在小時候,許下諾言要當無國界醫生的志願,還是回家玩NDS讓《超執刀》的月森醫生來實現吧。
Monday 9 September 2013
煌めく瞬間に捕われて
从朦胧的梦中醒来 阳光照射的早晨 一边听着喜欢的曲子 一边穿上洗干净的衬衫 昨晚那家伙 声嘶力竭 "想要刺激" "打破现状" 请别堕落 捕捉辉煌的瞬间 梦里也这样想 后悔的话 不讨人喜欢 流出的眼泪 如果美丽的话 是因为人又在无尽的旅程 花费了光阴 今日渐渐远去 快走到驿站吧 没看惯的景色 高兴地 进入拐角的古服店 从与往常不同的角度 窥视镜子 一定在那里看到新的什么 捕捉辉煌的瞬间 梦里也这样想 后悔的话 不讨人喜欢 流出的眼泪 如果美丽的话 是因为人又在无尽的旅程 花费了光阴 捕捉辉煌的瞬间 梦里也这样想 后悔的话 不讨人喜欢 流出的眼泪 如果美丽的话 是因为人又在无尽的旅程 花费了光阴
Endless Chain
无疑问我只爱你 虽然好像没有承诺 迷失和困惑 四处碰壁 因为我想永远和你在一起 现在你开始在迷惑的爱中融化 注视着模糊不清的明天 每天的时光稍纵即逝 我们不孤独 我们将一起踏上无尽的征途 I believe in our ENDLESS CHAIN 生命不能永恒 也许什么都不会改变 两个人的脚印不愿分开 我要紧拥着你 我们将一起踏上无尽的征途 I believe in our ENDLESS CHAIN
君が好きだと叫びたい
好想大叫我喜歡你
頂著耀眼的陽光 在街頭奔跑著
你像平時一樣地拍打我的肩頭
我毫無理由地迷戀上你
你從來不曾挽上我手臂
不知從何時開始 我的眼睛總是追尋著你
不能離開 無法搖動 Crazy for you
※
好想大聲說我喜歡你 試著去改變明天
想打破逐漸凍結的時間
我好想大聲說我喜歡你 鼓起勇氣踏出第一步吧
希望你能接受我這熱切的思念
嘎嘎作響的地板 越過熱鬧的桌間
我無意間沈醉在你的眼神裡
彷彿正在戀愛一般 為你而劇烈跳動的心
激烈地跳動 已不再說謊
到底要到何時 才能改變這令人厭煩的朋友關係
無法得到 我想確認 I take you away
好想大聲說我喜歡你 什麼我都不管了
我只想找出能融化你心的話
好想大聲說我喜歡你 今天我不回家
讓我們結束僅僅彼此注視的日子吧
I wanna cry for you
※ REPEAT
I wanna cry for you
頂著耀眼的陽光 在街頭奔跑著
你像平時一樣地拍打我的肩頭
我毫無理由地迷戀上你
你從來不曾挽上我手臂
不知從何時開始 我的眼睛總是追尋著你
不能離開 無法搖動 Crazy for you
※
好想大聲說我喜歡你 試著去改變明天
想打破逐漸凍結的時間
我好想大聲說我喜歡你 鼓起勇氣踏出第一步吧
希望你能接受我這熱切的思念
嘎嘎作響的地板 越過熱鬧的桌間
我無意間沈醉在你的眼神裡
彷彿正在戀愛一般 為你而劇烈跳動的心
激烈地跳動 已不再說謊
到底要到何時 才能改變這令人厭煩的朋友關係
無法得到 我想確認 I take you away
好想大聲說我喜歡你 什麼我都不管了
我只想找出能融化你心的話
好想大聲說我喜歡你 今天我不回家
讓我們結束僅僅彼此注視的日子吧
I wanna cry for you
※ REPEAT
I wanna cry for you
Monday 12 August 2013
赤いスイートピー
Take me to the beach
On a spring-coloured train
I'll snuggle up to your shirt that smells of cigarettes
Why is it that it's been half a year
Since we met
And you still
Won't even hold my hand?
I will follow you
I want to follow you
I will follow you
Even though I'm a bit weak-willed
Because you're a wonderful person
My red sweet pea
Growing on the shore of my heart
Sitting together on the bench
At the station in the spring rain
There's no sign of anyone else
And suddenly things get awkward
Why is it that every time
You glance at your watch
I feel like crying?
I will follow you
In winged boots
I will follow you
I want to run
Through the same youth as you
The bud by the tracks
Is a red sweet pea
I love you more than anyone else
I've ever met
I will follow you
I love the way you live
I cant go home (I cant go home) like this
The day spring came in my heart
A red sweet pea grew
On a spring-coloured train
I'll snuggle up to your shirt that smells of cigarettes
Why is it that it's been half a year
Since we met
And you still
Won't even hold my hand?
I will follow you
I want to follow you
I will follow you
Even though I'm a bit weak-willed
Because you're a wonderful person
My red sweet pea
Growing on the shore of my heart
Sitting together on the bench
At the station in the spring rain
There's no sign of anyone else
And suddenly things get awkward
Why is it that every time
You glance at your watch
I feel like crying?
I will follow you
In winged boots
I will follow you
I want to run
Through the same youth as you
The bud by the tracks
Is a red sweet pea
I love you more than anyone else
I've ever met
I will follow you
I love the way you live
I cant go home (I cant go home) like this
The day spring came in my heart
A red sweet pea grew
Tuesday 6 August 2013
Sweet Dream
It's gonna be another day with the sunshine
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
Your lips slightly brush against my cheeks, as you whisper that you love me
Inside my head is the morning coffee, am I dreaming?
It's gonna be another day with the sunshine
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
When we can get together I feel paradise
There's nothing that can make me happier than this,
Yes, that has to be right
Because right now you are by my side
My name, because it was so common, even I didn't like it
But when you call me, I only think of it prettily
It's gonna be another day with the sunshine
You make me feel beautiful, will you please hold me
Will you tell me that you love me...
When we can get together I feel paradise
Because finally, I feel like the main character in the movies,
Receiving love
I was reborn in your heart
Right this moment, there can't be anyone happier than me
Please don't make it break, don't break it, don't tell me I'm dreaming...
It's gonna be another day with the sunshine
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
When we can get together I feel paradise
There's nothing that can make me happier than this,
Yes, that has to be right
Because right now you are by my side
It's gonna be another day with the sunshine
You make me feel beautiful, will you please hold me
Will you tell me that you love me...
When we can get together I feel paradise
Because finally, I feel like the main character in the movies,
Receiving love
I was reborn in your heart
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
Your lips slightly brush against my cheeks, as you whisper that you love me
Inside my head is the morning coffee, am I dreaming?
It's gonna be another day with the sunshine
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
When we can get together I feel paradise
There's nothing that can make me happier than this,
Yes, that has to be right
Because right now you are by my side
My name, because it was so common, even I didn't like it
But when you call me, I only think of it prettily
It's gonna be another day with the sunshine
You make me feel beautiful, will you please hold me
Will you tell me that you love me...
When we can get together I feel paradise
Because finally, I feel like the main character in the movies,
Receiving love
I was reborn in your heart
Right this moment, there can't be anyone happier than me
Please don't make it break, don't break it, don't tell me I'm dreaming...
It's gonna be another day with the sunshine
The suns rays shine brightly outside of my window,
When my half-opened eyes are finally opened
I invision your face and it welcomes me...
When we can get together I feel paradise
There's nothing that can make me happier than this,
Yes, that has to be right
Because right now you are by my side
It's gonna be another day with the sunshine
You make me feel beautiful, will you please hold me
Will you tell me that you love me...
When we can get together I feel paradise
Because finally, I feel like the main character in the movies,
Receiving love
I was reborn in your heart
Saturday 20 July 2013
此情只待成追憶
男:為何思想昨天 仍然心中眷戀
時候換過了 但情未了 仍似永遠未完
合:為何跟你結識 如苦苦的壓迫
曾熱烈愛過 亦平淡過 長途有你共我
男:但我相信 愛是燃盡所有 就算今生不可永久
合:若你需要 我願陪在咫尺
男:但我知道情緣盡了
女:從未信相戀到白頭 沒有永遠的伴侶
合:若愛要犧牲 也要甘心 無人能妄說自由
男:為何思想昨天 女:相信可永久
男:仍然在心中眷戀 女:只有情義
男:曾浪漫愛過 也有風波
男:長途有你共我 女:長留你我心窩
男:沒有 女:沒有
男:虧欠 女:相欠
合:愛是原諒寬恕
男:就算所愛不可結果 女:永不說為何當初
男:願我所愛 女:但我所愛
男:如夢想中所愛 女:卻已不再似夢裡所愛對待我
男:或許你心從未屬我
合:茫茫心境似水 柔情不可再追
時候換過了 熱情未退 誰是最愛伴侶
你的心可會知 我的心未可制止
沉默地歎氣 未忘掉你 卻一生都想你
女:無論在那裡 願仍可以 會一生記念你
男:無論在那裡 願仍可以 合:會一生掛念 你
時候換過了 但情未了 仍似永遠未完
合:為何跟你結識 如苦苦的壓迫
曾熱烈愛過 亦平淡過 長途有你共我
男:但我相信 愛是燃盡所有 就算今生不可永久
合:若你需要 我願陪在咫尺
男:但我知道情緣盡了
女:從未信相戀到白頭 沒有永遠的伴侶
合:若愛要犧牲 也要甘心 無人能妄說自由
男:為何思想昨天 女:相信可永久
男:仍然在心中眷戀 女:只有情義
男:曾浪漫愛過 也有風波
男:長途有你共我 女:長留你我心窩
男:沒有 女:沒有
男:虧欠 女:相欠
合:愛是原諒寬恕
男:就算所愛不可結果 女:永不說為何當初
男:願我所愛 女:但我所愛
男:如夢想中所愛 女:卻已不再似夢裡所愛對待我
男:或許你心從未屬我
合:茫茫心境似水 柔情不可再追
時候換過了 熱情未退 誰是最愛伴侶
你的心可會知 我的心未可制止
沉默地歎氣 未忘掉你 卻一生都想你
女:無論在那裡 願仍可以 會一生記念你
男:無論在那裡 願仍可以 合:會一生掛念 你
Thursday 11 July 2013
Janice - Long distance
when i'm feeling blue
lost without a clue
sparks between our eyes
nothing can be as true
sing my life for you
paintings that i drew
one plus one makes two
how i wish to caress you
even though we're far apart
send my love with all my heart
when you miss me at night
look at the stars shining bright
for the time you pulled me through
all the things i do for you
running tears from my eyes
thinking how will i survive next goodbye
lost without a clue
sparks between our eyes
nothing can be as true
sing my life for you
paintings that i drew
one plus one makes two
how i wish to caress you
even though we're far apart
send my love with all my heart
when you miss me at night
look at the stars shining bright
for the time you pulled me through
all the things i do for you
running tears from my eyes
thinking how will i survive next goodbye
Sunday 7 July 2013
Valentine
If there were no words
No way to speak
I would still hear you
If there were no tears
No way to feel inside
I'd still feel for you
And even if the sun refuse to shine
Even if romance ran out of rhyme
You would still have my heart
Until the end of time
You're all i need
My love, my valentine
All of my life
I have been waiting for
All you give to me
You've opened my eyes
And showed me how to love unselfishly
I've dreamed of this a thousand times before
In my dreams I couldnt love you more
I will give you my heart
Until the end of time
You're all i need
My love, my valentine
And even if the sun refuse to shine
Even if romance ran out of rhyme
You would still have my heart
Until the end of time
Cuz all i need
Is you, my valentine
You're all i need
My love, my valentine
No way to speak
I would still hear you
If there were no tears
No way to feel inside
I'd still feel for you
And even if the sun refuse to shine
Even if romance ran out of rhyme
You would still have my heart
Until the end of time
You're all i need
My love, my valentine
All of my life
I have been waiting for
All you give to me
You've opened my eyes
And showed me how to love unselfishly
I've dreamed of this a thousand times before
In my dreams I couldnt love you more
I will give you my heart
Until the end of time
You're all i need
My love, my valentine
And even if the sun refuse to shine
Even if romance ran out of rhyme
You would still have my heart
Until the end of time
Cuz all i need
Is you, my valentine
You're all i need
My love, my valentine
Saturday 6 July 2013
Immunodiffusion
| http://210.36.18.48/gxujingpin/dwwswx/im/7.htm CHAPTER 7 ANTIGEN-ANTIBODY INTERACTIONS INTRODUCTION Antibodies constitute the humoral arm of acquired immunity that provides protection against infectious organisms and their toxic products. Therefore, the interaction between antigen and antibody is of paramount importance. In addition, because of the exquisite specificity of the immune response, the interaction between antigen and antibody in vitro is widely used for diagnostic purposes, for the detection and identification of either antigen or antibody. The utilization of the in vitro reaction between antigen and serum antibodies is termed serology. An example of the use of serology for the identification and classification of antigens is the serotyping of various microorganisms by the use of specific antisera. The interaction of antigen with antibodies may result in a variety of consequences, including precipitation (if the antigen is soluble), agglutination (if the antigen is particulate), and activation of complement. All of these outcomes are caused by the interactions between multivalent antigens and antibodies that have at least two combining sites per molecule. The consequences of antigen-antibody interaction listed above do not represent the primary interaction between antibodies and a given epitope but, rather, depend on secondary phenomena, which result from the interactions between multivalent antigens and antibodies. Such phenomena as the formation of precipitate, agglutination, and complement activation would not occur if the antibody with two or more combining sites reacted with a hapten (i.e., a unideterminant, univalent antigen), nor would they occur as a result of the interaction between a univalent fragment of antibody, such as Fab, and an antigen, even if the antigen is multivalent. The reasons for these differences are depicted in Figure 7.1A-E. Cross-linking of various antigen molecules by antibody is required for precipitation, agglutination, or complement activation, and it is possible only if the antigen is multivalent and the antibody is divalent [either intact, or F(ab')2] (see Fig. 7.lB, D, E). In contrast, no cross-linking is possible ifthe antigen or the antibody is univalent (Fig. 7. lA, C). There are many serologic reactions that demonstrate the binding between antigen and antibodies. This chapter describes selected reactions that are used in diagnosis; many others, not included here, are mostly variations of the reactions described here. PRIMARY INTERACTIONS BETWEEN ANTIBODY AND ANTIGEN No covalent bonds are involved in the interaction between antibody and an epitope. Consequently, the binding forces are relatively weak. They consist mainly of van der Waals forces, electrostatic forces, and hydrophobic forces, all of which require a very close proximity between the interacting moieties. Thus the interaction requires a very close fit between an epitope and the antibody, a fit that is often compared to that between a lock and a key. Because of the low levels of energy involved in the interaction between antigen and antibody, antigen-antibody complexes can be readily dissociated by low or high pH, by high salt concentrations, or by chaotropic ions, such as cyanates, which efficiently interfere with the hydrogen bonding of water molecules. Association Constant The reaction between an antibody and an epitope of an antigen is exemplified by the reaction between antibody and a univalent hapten. Because an antibody molecule is symmetric, with two identical Fab antigen combining sites, one antibody molecule binds with two identical hapten molecules, each Fab binding in an independent fashion with one hapten molecule. Affinity and Avidity The intrinsic association constant that characterizes the binding of an antibody with an epitope or a hapten is termed affinity. When the antigen consists of many repeating identical epitopes or when antigens are multivalent, the association between the entire antigen molecule and antibodies depends not only on the affinity between each epitope and its corresponding antibody but also on the sum of the affinities of all the epitopes involved. For example, the affinity of binding of antiA with multivalent A (shown in Fig. 7. lB) may be four or five orders of magnitude higher than between the same antibody (i.e., anti-A) and univalent A (Fig. 7. lA). This is because the pairing of anti-A with A (where A is multivalent) is influenced by the increased number of sites on A with which anti-A can react. While the term affinity denotes the intrinsic association constant between antibody and a univalent ligand such as a hapten, the term avidity is used to denote the overall binding energy between antibodies and a multivalent antigen. Thus, in general, IgM antibodies are of higher avidity than IgG antibodies, although the binding of each Fab in the IgM antibody with ligand may be of the same affinity as that of the Fab from IgG. SECONDARY INTERACTIONS BETWEEN ANTIBODY AND ANTIGEN Agglutination Reactions  the reactions of antibody with a multivalent antigen that is particulate (i.e., an insoluble particle) results in the cross-linking of the various antigen particles by the antibodies. This cross-linking eventually results in the clumping or agglutination of the antigen particles by the antibodies. TITER. The agglutination of an antigen as a result of cross-linking by antibodies is dependent on the correct proportion of antigen to antibody. Figure 7.2 depicts an example of an agglutination test for antibodies to the bacterium Brucella abortus present in the serum of an infected individual. The figure shows 10 tubes containing twofold serial dilution of the serum, ranging from 1:4 to 1:2048, to which equal amounts of a suspension of B. abortus (a particulate antigen) are added. The plus and minus signs denote the presence or absence of agglutination. The results of the test (shown below each tube) indicate that agglutination occurs at dilutions of serum of 1:16 to 1:1024. There is no agglutination at higher dilutions because at such dilutions there are not enough antibodies to cause appreciable, visible agglutination. The highest dilution of serum that still causes agglutination, but beyond which no agglutination occurs, is termed the titer. PROZONE. shows tubes with no agglutination, although they contain a suspension of antigen and concentrated serum (diluted only 1:4 or 1:8). It is a common observation that agglutination may not occur at high concentrations of antibody, even though it does take place at higher dilutions of serum. The tubes with high concentrations of serum, where agglutination does not occur, represent a prozone. In the prozone, antibodies are present in excess. Agglutination may not occur at high ratio of antibody to antigen because every epitope on one particle may bind only to a single antibody molecule, preventing cross-linking between different particles (see Fig. 7.3). Because of the prozone phenomenon, in testing for the presence of agglutinating antibodies to a certain antigen, it is imperative that the antiserum be tested at several dilutions. Testing serum at only one concentration may give misleading conclusions if no agglutination occurs, because the absence of agglutination might reflect either a prozone or a lack of antibody. The agglutinating titer of a certain serum is only a semiquantitative expression of the antibodies present in the serum; it is not a quantitative measure of the concentration of antibody (weight/volume). Rather, the titer represents the ability of a certain dilution (i.e., volume) of the serum containing the antibodies to cause agglutination. As such, the titer of a given antisermn may be used for comparison with the agglutinating titer to another antiserum to the same antigen. For example, a change in titer of anti-B, abortus antibodies, in an individual, from 1:4 to 1:1024 would indicate an acute infection, while on the other hand a drop in titer might suggest that antimicrobial therapy was working. Thus, agglutination titers are useful for comparisons of the relative concentrations of agglutinating antibodies in various sera specific for the same antigen. Since the titer in any agglutination assay depends on a variety of factors, such as size, charge, and density of epitopes on an antigen, it is of little use to compare titers of antisera to different antigens. ZETA POTENTIAL. The surfaces of certain particulate antigens may possess an electrical charge, as, for example, the net negative charge on the surface of red blood cells caused by the presence of sialic acid. When such charged particles are suspended in saline solution, an electrical potential termed the zeta potential is created between particles, preventing them from getting very close to each other. This introduces a difficulty in agglutinating charged particles by antibodies, in particular red blood cells by IgG antibodies. The distance between the Fab arms of the IgG molecule, even in its most extended form, is too short to allow effective bridging between two red blood cells across the zeta potential. Thus, although IgG antibodies may be directed against antigens on the charged erythrocyte, agglutination may not occur because of the repulsion by the zeta potential. On the other hand, some of the Fab areas oflgMpentamers are far enough apart and can bridge red blood cells separated by the zeta potential. This property of IgM antibodies, together with their pentavalence, is a major reason for their effectiveness as agglutinating antibodies. Through the years attempts were made to improve agglutination reactions by decreasing the zeta potential in various ways, none of which was universally applicable or effective. However, an ingenious method was devised in the 1950s by Coombs to overcome this problem. This method, described below, facilitates the agglutination of erythrocytes by IgG antibodies specific for erythrocyte antigens. It is also useful for the detection of antibodies that are present on the surface of erythrocytes but that are unable to agglutinate them. PASSIVE AGGLUTINATION. The agglutination reaction can be used with particulate antigens (e.g., erythrocytes or bacteria) and also with soluble antigens, provided that the soluble antigen can be firmly attached to insoluble particles. For example, the soluble antigen thyroglobulin can be attached to latex particles, so that the addition of antibodies to the thyroglobulin antigen will cause agglutination of the latex particles coated with thyroglobulin. Of course, the addition of soluble antigen to the antibodies prior to the introduction of the thyroglobulin cortex latex particles will inhibit the agglutination because the antibodies will first combine with the soluble antigen, and if the soluble antigen is present in excess, the antibodies will not be able to bind with the particulate antigen. This latter example is referred to as agglutination inhibition. It should be distinguished from agglutination inhibition in which antibodies to certain viruses inhibit the aggluti nation of red blood cells by the virus. In these cases, the antibodies are directed to the area or areas on the virus that bind with the appropriate virus receptors on the red blood cells. When the antigen is a natural constituent of a particle, the agglutination reac tion is referred to as direct agglutination. When the agglutination reaction takes place between antibodies and soluble antigen that had been attached to an insolu ble particle, the reaction is referred to as passive agglutination. The agglutination reaction (direct or passive, either employing or not employ ing the Coombs test) is widely used clinically. In addition to the examples already given, major applications include erythrocyte typing in blood banks, diagnosis of various immunologically mediated hemolytic diseases, such as drug-induced auto hemolytic anemia, tests for rheumatoid factor (human IgM anti-human IgG), con firmatory test for syphilis, and the latex test for pregnancy, which involves the de tection of human chorionic gonadotropin (HCG) in the urine of pregnant women.  Precipitation Reaction REACTION IN SOLUTIONS. In contrast to the agglutination reaction, which takes place between antibodies and particulate antigen, the precipitation reaction takes place when antibodies and soluble antigen are mixed. As in the case of agglutination, precipitation of antigen-antibody complexes occurs because the divalent antibody molecules cross-link multivalent antigen molecules to form a lattice. When it reaches a certain size, this antigen-antibody complex loses its solubility and precipitates out of solution. The phenomenon of precipitation is termed the precipitin reaction. When increasing concentrations of antigen are added to a series of tubes that contain a constant concentration of antibodies, variable amounts of precipitate form. The weight of the precipitate in each tube may be determined by a variety of methods. If the amount of the precipitate is plotted against the amount of antigen added, a precipitin curve like the one shown in Figure 7.6 is obtained. There are three important areas under the curve shown in Figure 7.6: (1) the zone of antibody excess, (2) the equivalence zone, and (3) the zone of antigen excess. In the equivalence zone, the proportion of antigen to antibody is optimal for maximal precipitation; in the zones of antibody excess or antigen excess, the proportions of the reactants do not lead to efficient cross-linking and formation of precipitate. It should be emphasized that the zones of the precipitin curve are based on the amount of antigen-antibody complexes precipitated. However, the zones of antigen or antibody excess may contain soluble antigen-antibody complexes, particularly the zone of antigen excess where a minimal amount of precipitate is formed, but large amounts of antigen--antibody complexes are present in the supematant. Thus, the amount of precipitate formed is dependent on the proportions of the reactant antigens and antibodies: the correct proportion of the reactions result in maximal formation of precipitate; excess of antigen (or antibody) results in soluble complexes. PRECIPITATION REACTIONS IN GELS. Precipitation reactions between soluble antigens and antibodies can take place not only in solution but also in semisolid media such as agar gels. When soluble antigen and antibodies are placed in wells cut in the gel (Fig. 7.7A), the reactants diffuse in the gel and form gradients of concentration, with the highest concentrations closest to the wells. Somewhere between the two wells, the reacting antigen and antibodies will be present at proportions that are optimal for formation of a precipitate. If the antibody well contains antibodies 1, 2, and 3 specific for antigens 1, 2, and 3, respectively, and if antigens 1, 2, and 3, placed in the antigen well, diffuse at different rates (with diffusion rates of 1 >> 2 >> 3), then three distinct precipitin lines will form. These three precipitin lines form because anti-1, anti-2, and anti3, which diffuse at the same rate, react independently with antigens 1, 2, and 3, respectively, to form three equivalence zones and thus three separate lines of precipitate (Fig. 7.7B). Different rates of diffusion of both antibody and antibody and antigen result from differences in concentration, molecular size, or shape. This double-diffusion method, developed by Ouchterlony, where antigen and antibody diffuse toward each other, is very useful for establishing the antigenic relationship between various substances, as shown in Figure 7.8. Three reaction patterns are seen in gel diffusion, each of which is illustrated in Figure 7.8: patterns of identity, patterns of nonidentity, and patterns of partial identity. Patterns of Identity. In the example given on the left in Figure 7.8, the central well contains antibodies and the peripheral wells contain identical antigens. The antibodies diffuse from the central well toward the antigens that, since they are identical, form one continuous, coalescing precipitin line. This pattern, formed when the two antigens are identical, is termed a pattern of identity. Patterns of Nonidentity. In the example in the center of Figure 7.8, the central well contains antibodies to antigen 1 and antibodies to antigen 2, two nonrelated antigens, and the peripheral wells contain the two nonrelated antigens, antigen 1 and antigen 2. The two antibody (immunoglobulin)populations diffuse toward the peripheral wells. Antigen 1 and antigen 2 diffuse from the two peripheral wells toward the antibodies. Each antigen forms an independent precipitin line with its corresponding antibody at an equivalent point. The precipitin lines cross each other since each antigen diffuses across the band formed by the other antigen until it meets its specific antibody diffusing toward it. A pattern where the precipitin lines cross each other denotes nonidentity of the two antigens. Patterns of Partial Identity. The pattern of partial identity is shown in the fight-hand portion of Figure 7.8, where the center well contains antibodies to various epitopes of antigen 1. The reaction of these antibodies with antigen 1 results in a precipitin line. Antigen 2, however, contains some (but not all) of the epitopes present on antigen 1. Thus, some of the antibodies to antigen 1 will also combine with antigen 2. This partial identity between the two antigens is responsible for the coalescence of the two lines to give a line of identity. However, antibodies that do not bind with antigen 2 will pass through this line of precipitate, combine with antigen 1 on the other side, and form a spur. This pattern, with the formation of a spur, denotes partial identity, signifying that antigen 1 and antigen 2 share epitopes, with antigen 1 having more epitopes (and being able to react with more antibody populations) than antigen 2.  RADIAL IMMUNODIFFUSION. The radial immunodiffusion test, represents a variation of the diffusion test. The wells contain antigen at different concentrations, while the antibodies are distributed uniformly in the agar gel. Thus, the precipitin line is replaced by a precipitin ring around the well. The distance the precipitin ring migrates from the center of the antigen well is directly proportional to the concentration of antigen in the well. The relationship between concentration of antigen in a well and the diameter of the precipitin ring can be plotted .If wells, such as F and G, contain unknown amounts of the same antigen, the concentration of that antigen in these wells can be determined by comparing the diameter of the precipitin ring with the diameter of the ring formed by a known concentration of the antigen. An important application of radial immunodiffusion is its use clinically to measure concentrations of serum proteins. To do so, antiserum to various serum proteins is incorporated in the gel; concentration of a particular protein in a serum sample is determined by comparing the diameter of the resulting precipitin ring with the diameter obtained by known concentrations of the protein in question. IMMUNOELECTROPHORESIS.Immunoelectrophoresis involves separating a mixture of proteins in an electrical field (electrophoresis) followed by their detection with antibodies diffusing into the gel. It is very useful for the analysis of a mixture of antigens by antiserum that contains antibodies to the antigens in the mixture. For example, in the clinical characterization of human serum proteins, a small drop of human serum is placed in a well cut in the center of a slide that is coated with agar gel. The serum is then subjected to electrophoresis, which separates the various components according to their mobilities in the electrical field. After electrophoresis, a trough is cut along the side of the slides, and antibodies to human serum proteins are placed in the trough. The antibodies diffuse in the agar, as do the separated serum proteins. At an optimal antigen:antibody ratio for each antigen and its corresponding antibodies, they form precipitin lines. The result is a pattern similar to that depicted in Figure 7.10. Comparison of the pattern and intensity of lines of normal human serum with the patterns and intensity of lines obtained with sera of patients may reveal an absence, overabundance, or other abnormality of one or more serum proteins.  WESTERN BLOTS (IMMUNOBLOTS). In the Western blot (immunoblot) technique, antigen (or a mixture of antigens) is first separated in gel. The separated material is then "blotted" omo nitrocellulose sheets to which the antigen binds strongly. Amibody, which is then applied to the nitrocellulose sheet, binds with its specific antigen. The antibody may be labeled (e.g., with radioactivity), or a labeled anti-immunoglobulin may be used to localize the antibody and the antigen to which the first antibody is bound. These so-called "Western blots" are gaining wide application in research and clinical laboratories for the characterization of antigen.  IMMUNOASSAYS Direct Binding Immunoassays Radioimmunoassay (RIA) employs isotopically labeled molecules and permits measurements of extremely small amounts of antigen, antibody, or antigen-antibody complexes. The concentration of such labeled molecules is determined by measuring their radioactivity, rather than by chemical analysis. The sensitivity of detection is thus increased by several orders of magnitude. For the development of this highly sensitive analytical method that has tremendous applications in hormone assays as well as assays of other substances of biological importance, RosalynYalow received the Nobel Prize. The principle of radioimmunoassay is illustrated in Figure 7.11A,B. A known amount of radioactively labeled antigen is reacted with a limited amount of antibody. The solution now contains antibody-bound labeled antigen, as well as some unbound labeled antigen. After separating the antigen bound to antibody from free antigen, the amount of radioactivity bound to antibody is determined (Fig. 7.1 lA). The test continues with performance of a similar procedure in which the same amount of labeled antigen is premixed with unlabeled antigen (Fig. 7.1 lB). The mixture is reacted with the same amount of antibody as before, and the antibody-bound antigen is separated from the unbound antigen. The unlabeled antigen competes with the labeled antigen for the antibody and, as a result, less label is bound to antibody than in the absence of unlabeled antigen. The more unlabeled antigen present in the reaction mixture, the smaller the ratio of antibodybound, radiolabeled antigen to free, radiolabeled antigen. This ratio can be plotted as a function of the concentration of the unlabeled antigen used for competition. To determine an unknown concentration of antigen in a solution, a sample of the solution is mixed with predetermined amounts of labeled antigen and antibody. The ratio of bound/free radioactivity is compared with that obtained in the absence of unlabeled antigen (the latter value is set at 100%). An important step in performing a radioimmunoassay, as described above, is the separation of free antigen from that bound to antibody. Depending upon the antigen, this separation can be achieved in a variety of ways, principal among which is the anti-immunoglobulin procedure. The anti-immunoglobulin procedure is based on the fact that antigen (labeled or unlabeled) bound to immunoglobulin will also be precipitated, following the addition of anti-immunoglobul!n, antibodies, so that only unbound antigen remains in the supematant. Radlolmmunoassays commonly employ rabbit antibodies to the desired antigens. These rabbit antibody-antigen complexes may be precipitated by the addition of goat antibodies raised against rabbit immtmoglobulins. Since the amounts of antigen and antibody required for radioimmunoassay are extremely small, the antigen-antibody complexes reacted with anti-immunoglobulin would form only tiny precipitates. It is difficult, if not impossible, to recover these precipitates quantitatively by conventional means, in order to determine their radioactivity. To overcome this problem, it is customary to add nonspecific immunoglobulins to the reaction mixture, thereby increasing the amount of total immunoglobulins to an amount that can easily be precipitated by anti-immunoglobulins and recovered quantitatively. Such precipitates consist mainly of nonspecific immunoglobulins to which radioactive antigen does not bind. However, they also contain the extremely small amount of antigen-specific immunoglobulin and any radioactive antigen bound to it. An alternative method of separating complexes of antigen bound to antibody from free antigen is based on the fact that immunoglobulins become insoluble and precipitate in a solution containing 33% saturated ammonium sulfate. If the antigen does not precipitate in 33% ammonium sulfate, the addition of ammonium sulfate to 33% will cause the antibody complexed to antigen to precipitate, leaving the free antigen in solution. Here again, the amounts of antibodies reacting with antigen (or free antibodies) is so small, unable to form precipitates. As described for the radioimmunoassay where anti-immunoglobulins are used for the separation of antibody-bound antigen from free antigen, a sufficient amount of nonspecific immunoglobulins is added to the mixture so that an appreciable precipitate will form at 33% saturation ammonium sulfate to enable the separation of free antigen from antigen bound to antibody. Solid-Phase Immunoassays Solid-phase immunoassay is one of the most widely used immunologic techniques. It is now automated and is widely used in clinical medicine for the detection of antigen or antibody. Solid-phase immunoassays employ the property of various plastics (e.g., polyvinyl or polystyrene) to adsorb monomolecular layers of proteins onto their surface. Although the adsorbed molecules may lose some of their antigenic determinants, enough remain unaltered and can still react with their corresponding antibodies. The presence of these antibodies, bound to antigen adsorbed onto the plastic, may be detected by the use of anti-immunoglobulins (Fig. 7.13) labeled with a radioactive tracer or with an enzyme. A solid-phase assay that uses radioactive anti-immunoglobulins is termed a solid-phase radioimmunoassay (SPRIA). If the test uses anti-immunoglobulins that are labeled with an enzyme that can be detected by the appearance of a color on addition of proper substrate, the test is called an enzyme-linked immunosorbent assay (ELISA). Because of problems associated with disposal or radioactive waste and the cost of radiation measuring instruments, the ELISA is rapidly replacing SPRIA. It should be emphasized that after coating the plastic surface with antigen, it is imperative to "block" any uncoated plastic surface to prevent it from absorbing the other reagents, most importantly the labeled reagent. Such "blocking" is achieved by coating the plastic surface with a high concentration of an unrelated protein, such as gelatin, after the application of the antigen. Solid-phase immunoassay may be used to detect the presence of antibodies to the antigen that coats the plastic. Since the plastic wells are usually coated with relatively large amounts of antigen, the higher the concentration of antibodies bound with the antigen, the higher the amount of labeled anti-immunoglobulin that can bind to the antibodies. Thus, it is ~mportant always to use an excess of labeled anti-immunoglobulin to assure saturation. Solid-phase immunoassay may be used for the qualitative or quantitative determinations of antigen. Such determinations are performed by mixing the antiserum with varying known amounts of antigen before adding the antiserum to the antigen-coated plastic wells. This preliminary procedure results in the binding of the antibodies with the soluble antigen, decreasing the availability of free antibodies for binding with the antigen that is coating the plastic. The higher the concentration of the soluble antigen that reacts with antibodies prior to addition of the antibody to the wells, the lower the number of antibodies that can bind with the antigen on the plate, and the lower the number of labeled anti-immunoglobulin that can bind to these antibodies. The decrease in the amount of bound label as a function of the concentration of antigen used to cause this decrease can be plotted, and the amount of antigen in an unknown solution can then be determined from the graph by a comparison of the decrease in bound label caused by the unknown solution to the decrease caused by known concentrations of pure antigen.  IMMUNOFLUORESCENCE A fluorescent compound has the property of emitting light of a certain wave length when it is excited by exposure to light of a shorter wavelength. Immunoflu orescence 'is a method for localizing an antigen by the use of fluorescence-labeled antibodies. The procedure, originally described by Coons, employs antibodies to which fluorescent groups have been covalently linked without any appreciable change in antibody activity. One fluorescent compound that is widely used in immunology isfiuorescein isothiocyanate (FITC), which fluoresces with a visible greenish color when ex cited by ultraviolet light. FITC is easily coupled to free amino groups. Another widely used fluorescent compound is tetramethyl rhodamine isothiocyanate (TRITe), which fluoresces red-orange and is also easily coupled to free amino groups. Specially constructed microscopes permit visualization of fluorescent antibody on a microscopic specimen, and fluorescent antibodies are widely used to localize antigens on various tissues and microorganisms. There are two important and related procedures that employ fluorescent antibodies: direct immunofluorescence and indirect immunofluorescence. Direct Immunofluorescence Direct immunofluorescence is primarily for detection of antigen and involves reacting the target tissue (or microorganism) with fluorescently labeled specific antibodies. It is widely used clinically for identifying lymphocytic subsets and for demonstrating the presence of specific protein deposition in certain tissues such as kidney and skin in cases of systemic lupus erythematosus (SLE).  Indirect Immunofluorescence Indirect immunofluorescence involves first reacting the target with specific antibodies. This reaction is followed by subsequent reaction with fluorescently labeled anti-immunoglobulin. The indirect immunofluorescence method is more widely used than the direct method, because a single fluorescent anti-immunoglobulin antibody can be used to localize antibody of many different specificities. Moreover, since the antiimmunoglobulins contain antibodies to many epitopes on the specific immunoglobulin, the use of fluorescent anti-immunoglobulins amplifies manyfold the fluorescent signal.  FLUORESCENCE-ACTIVATED CELL-SORTING ANALYSIS A very powerful tool has been developed around the use of fluorescent antibody specific for cell-surface antigens. This is the technique of fluorescence-activated cell sorting (FACS). A cell suspension labeled with specific fluorescent antibody is passed through an apparatus that forms a stream of small droplets each containing one cell. These droplets are passed between a laser beam of ultraviolet light and a detector for picking up emitted fluorescence when a labeled cell is present in the droplet. This emitted signal is passed to an electrode that charges the droplet, leading to its deflection (Fig. 7.14) in an electromagnetic field. Thus as all droplets fall through they are collected and counted depending on whether they emit a signal. By sophisticated electronics the fluorescein-stained and unstained cells may be counted, as well as the intensity of fluorescence on each. With this type of apparatus it is now possible to rapidly develop a profile of a pool of lymphocytes based on their differential expression of cell-surface molecules, the relative amount of cell-surface molecule expressed on each cell, and the size distribution and numbers of each cell type. It is also possible to use the apparatus to sort a collection of cells stained with five or more different fluorescent labels and obtain a very homogeneous sample of a particular cell type. A variation of this technique uses fluorescent antibodies coupled to magnetic beads to separate cell populations. Cells which bind to the fluorescent antibody can be separated from unstained cells by a magnet. Both FACS and magnetic bead separation methods have resulted in the isolation of very rare cells such as stem cells (see Chapter 8). IMMUNOABSORPTION AND IMMUNOADSORPTION Because of the specific binding between antigen and antibody, it is possible to "trap," or selectively remove, an antigen against which an antibody is directed from a mixture of antigens in solution. By the same token, it is possible to trap or remove selectively the antigen-specific antibodies from a mixture of antibodies, using the specific antigen. There are two general methods by which this removal can be achieved. The methods are related, but, in one method, the absorption is done with both reagents in solution (immunoabsorption); in the second method, it is performed with one reagent attached to an insoluble support (immunoadsorption). Immunoadsorption is of particular value because the adsorbed material can be recovered from the complex by careful treatments that dissociate antigen-antibody complexes, such as lowering the pH (HCl-glycine or acetic acid, pH 2-3) or adding chaotropic ions. This enables one effectively to purify antigens or antibodies of specific interest. MONOCLONAL AND GENETICALLY ENGINEERED ANTIBODIES Monoclonal Antibodies The specificity of the immune response has served as the basis for serologic reactions in which antibody specificity is used for the qualitative and quantitative determination of antigen. The discriminating power of serum antibody is not without limitations, however, because the immunizing antigen, which usually has many epitopes, leads to production of antisera that contain a mixture of antibodies with varying specificity for all the epitopes. Indeed, even antibodies to a single epitope are usually mixtures of immunoglobulins with different fine specificities, and therefore different affinities for the determinant. Furthermore, immunization with an antigen expands various populations of antibody-forming lymphocytes (see Chapter 11). These cells can be maintained in culture for only a short time (on the order of days), so it is impractical, if not impossible, to grow normal cells and obtain clones that produce antibodies of a single specificity A quantum leap in the resolution and discriminating power of antibodies was provided in the 1970s with the developmem of methods for the generation of monoclonal antibodies by Milstein and K6hler, who shared the Nobel Prize for this development. Monoclonal antibodies are homogeneous populations of antibody molecules, derived from a single antibody-producing cell, in which all antibodies are identical and of the same precise specificity for a given epitope.  Milstein and Kohler took advantage of the properties of malignant plasma cells, which are "immortal" and can be maintained in culture for years. They selected a population of malignant plasma cells unable to secrete immunoglobulin and also deficient in the enzyme hypoxanthine phosphoribosyl transferase (HPRT) that would die unless HPRT were introduced. The malignant cells were then fused with antibody-producing cells, which have HPRT. The biotechnology for the production of hybridomas, developed by K6hler and Milstein, is illustrated in Figure 7.15. The fusion is generally accomplished by thc use of polyethylene glycol (PEG) or by inactivated Sendai virus. The nuclei of the hybrids also fuse, and the hybridoma cells then possess both the capacity to manufacture immunoglobulins and the ability to survive in culture in a select medium such as that containing hypoxanthine, aminopterin, and thymidine (HAT). The enzyme deficiency of the malignant cell results in its death in this medium unless that deficiency is corrected by the acquisition of the enzyme-producing genes derived from the antibody-producing cell. Thus, the hybrids can be separated from the contaminating malignant cells, which do not survive, because they have not acquired the enzyme. Those hybrid cells synthesizing specific antibody are selected by some test for antigen reactivity and then cloned from single cells and propagated in tissue culture, each clone synthesizing antibodies of a single specificity. These highly specific, monoclonal antibodies are used as reagents for numerous procedures, ranging from specific diagnostic tests to "magic bullets" in immunotherapy of cancer (see Chapter 21). In immunotherapy, various drugs or toxins are conjugated to monoclonal antibodies, which, in mm, "deliver" these substances to the tumor cells against which the antibodies are specifically directed. Genetically Engineered Antibodies To date most of the monoclonal antibodies are made in mouse cells. These are suitable for diagnostic and many other purposes. However, their administration into humans carries the complication that the patient will form antibodies to the mouse immunoglobulins. Attempts to develop in vitro human monoclonal antibodies are by and large quite difficult. Human monoclonal antibodies are being currently produced by genetic engineering utilizing several approaches. One method utilizes the technology of recombinant DNA to produce a chimeric monoclonal antibody. This molecule consists of the constant region of human immunoglobulin and a variable region of a mouse immunoglobulin.  A more recent technology utilizes the polymerase chain reaction (PCR) to generate gene libraries of heavy and light chains from DNA obtained from hybridoma cells or plasma cells, joining at random numerous heavy and light chains and screening the resulting Fab clones for antibody activity against a desired antigen. With this technology it is now possible to produce millions of clones of different specificities, to rapidly screen them for the desired specificity and generate the desired monoclonal Fab constructs without immunization and without the difficulties encountered with the production of monoclonal antibodies, especially human monoclonal antibodies. Although this chapter deals with antibodies, it seems appropriate to mention at this point that the hybridoma technology is not limited to the production of monoclonal immunoglobulins. In the late 1970s, methods for producing hybridomas were also developed for T cells, fusing lines of malignant T cells with nonmalignant, antigen-specific T lymphocytes whose populations have been expanded by immunization with antigen. T-cell hybridomas have been very useful for studying the relationship between T cells of a single specificity with their corresponding epitope. SUMMARY 1. The reaction between an antibody and an epitope does not involve covalent forces; it involves weak forces of interaction such as electrostatic, hydrophobic, and van der Waals forces. Consequently, for a significant interaction, the antibody combining site and the epitope require a close steric fit like a lock and key. 2. Only the reaction between a multivalent antigen and at least a divalent antibody can bring about secondary antigen-antibody reactions that depend on cross-linking of antigen molecules by antibodies. These secondary reactions do not take place with haptens or monovalent Fab. 3. The interaction between a soluble antibody and an insoluble particulate antigen results in agglutination. The extent of agglutination depends on the proportions o.f the interacting antibody and antigen. At high antibody levels, excess agglutination may not occur. Th~s is referred to as a prozone. The term titer refers to the highest serum dilution at which agglutination still takes place and beyond which, at higher dilution, no agglutination occurs. 4. Because of the zeta potential, which precludes some antigenic particles from approaching each other closer than a certain distance, IgG antibodies may be incapable of causing agglutination. IgM antibodies, however, can bind such distant particles and agglutinate them. 5. The use of heterologous anti-immunoglobulin antibodies may bridge between antigenic particles that are bound to nonagglutinating antibodies, leading to agglutination. This sequence of events is the basis for the Coombs test. 6. Precipitation reactions occur on mixing, at the right proportions, of soluble multivalent antigen and (at least) divalent antibodies. The precipitation reaction may take place in aqueous media or in gels. 7. The reaction in gels, between soluble antigen and antibodies, may be used for the qualitative and quantitative analysis of antigen or antibody. Ex amples are gel diffusion tests, radial diffusion tests, and immunoelectrophoresis. 8. Radioimmunoassay is a test that is based on competitive inhibition of nonlabeled and labeled antigen for antibody (in antibody deficiency). Antibody-bound antigen must be separated from nonbound labeled antigen. Separation is usually achieved by precipitation with anti-immunoglobulins. 9. Solid-phase immunoassay is a test that employs the property of many proteins to adhere to plastic and form a monomolecular layer. Antigen is applied to plastic wells, antibodies are added, the well is washed, and any antibodies bound to antigen are measured by the use of radiolabeled or enzyme-linked anti-immunoglobulins. 10. ELISA. This enzyme-linked immunosorbent assay is essentially a solid-phase immunoassay in which an enzyme is linked to the anti-immunoglobulin. Quantitation of enzyme-linked anti-immunoglobulins is achieved by colorimetric evaluation, after the addition of a substrate, which changes color on the action of the enzyme. 11. Immunofiuorescence. In immunofiuorescence, the antigen is detected by the use of fluorescence-labeled immunoglobulins. In direct immunofiuorescence, the antibody to the antigen in question carries a fluorescent label. In indirect immunofiuorescence, the antigen-specific antibody is not labeled; it is detected by the addition of fiuorescently labeled anti-immunoglobulin. 12. Monoclonal antibodies are highly specific reagents consisting of homogeneous populations of antibodies, all of precisely the same specificity toward an epitope. |
Tuesday 2 July 2013
The value of Youth
When you are young you don't see youth. When you see youth, it means only one thing.
Monday 17 June 2013
這些英文千萬別不懂裝懂
◆Lover 情人(不是:愛人)
◆Sporting house 妓院(不是:體育室)
◆Dead president 美鈔(不是:死了的總統)
◆Service station 加油站(不是:服務站)
◆Rest room 廁所(不是:休息室)
◆Busboy 餐館勤雜工(不是:公汽售票員)
◆Dry goods 紡織品 穀物(不是:乾貨)
◆Heart man 換心人(不是:有心人)
◆Tea shop 茶館;小吃店(不是:茶葉店)
◆Senior citizen 老年人(不是:高級公民)
◆Wash one’s hands 上廁所(不是:洗手)
◆A busy body 愛管閒事的人(不是:忙人)
◆A black sheep 害群之馬(不是:一隻黑羊)
◆Be taken in 受騙,上當(不是:被接納)
◆Pull sb’s leg 開玩笑(不是:拖後腿)
◆African American 美國黑人(不是:非洲美國人)
◆Eat one’s words 收回前言,改正錯話(不是:食言)
◆Mad doctor 精神病科醫生(不是:發瘋的醫生)
◆Eleventh hour 最後時刻(不是:十一點)
◆Personal remark 人身攻擊(並非個人評論)
◆Sweet water 淡水(不是:糖水或者甜水)
◆Confidence man 騙子(不是:信得過的人)
◆Criminal lawyer 刑事律師(不是:犯罪的律師)
◆Dressing room 化粧室(不是:試衣間或者更衣室)
◆Horse sense 常識(不是:馬的感覺)
◆Capital idea 好主意(不是:資本主義思想)
◆Familiar talk 庸俗的交談(不是:熟悉的談話)
◆Black tea紅茶 (不是:黑茶)
◆Green hand 新手 (不是:綠手)
◆Have a fit 勃然大怒(不是:試穿)
◆Black art 妖術(不是:黑色藝術)
◆White coal (作動力來源用的)水
◆Chinese dragon 麒麟(不是:中國龍)
◆Red tape 官僚習氣(不是:紅色帶子)
◆China policy 對華政策(不是:中華政策)
◆White man 忠實可靠的人(不是:皮膚白色的人)
◆Black stranger 完全陌生的人(不是:陌生的黑人)
◆Eat ones words 收回前言(不是:食言)
◆An apple of love 番茄(不是:愛情之果)
◆Handwriting on the wall 不祥之兆(不是:大字報)
◆Bring down the house 博得滿堂喝彩(不是:推倒房子)
◆Blind date(由第三者安排的)男女初次見面(並非盲目的約會或者是瞎
約會)
◆Roadside business 汽車飯店;汽車旅館;汽車影院(不是:路邊店 )
◆American Dream 美國的生活方式,美國人的自由民主觀念(不是:美國夢)
◆Yellow book 黃皮書(法國政府報告書,以黃紙為封,不是:黃
◆Sporting house 妓院(不是:體育室)
◆Dead president 美鈔(不是:死了的總統)
◆Service station 加油站(不是:服務站)
◆Rest room 廁所(不是:休息室)
◆Busboy 餐館勤雜工(不是:公汽售票員)
◆Dry goods 紡織品 穀物(不是:乾貨)
◆Heart man 換心人(不是:有心人)
◆Tea shop 茶館;小吃店(不是:茶葉店)
◆Senior citizen 老年人(不是:高級公民)
◆Wash one’s hands 上廁所(不是:洗手)
◆A busy body 愛管閒事的人(不是:忙人)
◆A black sheep 害群之馬(不是:一隻黑羊)
◆Be taken in 受騙,上當(不是:被接納)
◆Pull sb’s leg 開玩笑(不是:拖後腿)
◆African American 美國黑人(不是:非洲美國人)
◆Eat one’s words 收回前言,改正錯話(不是:食言)
◆Mad doctor 精神病科醫生(不是:發瘋的醫生)
◆Eleventh hour 最後時刻(不是:十一點)
◆Personal remark 人身攻擊(並非個人評論)
◆Sweet water 淡水(不是:糖水或者甜水)
◆Confidence man 騙子(不是:信得過的人)
◆Criminal lawyer 刑事律師(不是:犯罪的律師)
◆Dressing room 化粧室(不是:試衣間或者更衣室)
◆Horse sense 常識(不是:馬的感覺)
◆Capital idea 好主意(不是:資本主義思想)
◆Familiar talk 庸俗的交談(不是:熟悉的談話)
◆Black tea紅茶 (不是:黑茶)
◆Green hand 新手 (不是:綠手)
◆Have a fit 勃然大怒(不是:試穿)
◆Black art 妖術(不是:黑色藝術)
◆White coal (作動力來源用的)水
◆Chinese dragon 麒麟(不是:中國龍)
◆Red tape 官僚習氣(不是:紅色帶子)
◆China policy 對華政策(不是:中華政策)
◆White man 忠實可靠的人(不是:皮膚白色的人)
◆Black stranger 完全陌生的人(不是:陌生的黑人)
◆Eat ones words 收回前言(不是:食言)
◆An apple of love 番茄(不是:愛情之果)
◆Handwriting on the wall 不祥之兆(不是:大字報)
◆Bring down the house 博得滿堂喝彩(不是:推倒房子)
◆Blind date(由第三者安排的)男女初次見面(並非盲目的約會或者是瞎
約會)
◆Roadside business 汽車飯店;汽車旅館;汽車影院(不是:路邊店 )
◆American Dream 美國的生活方式,美國人的自由民主觀念(不是:美國夢)
◆Yellow book 黃皮書(法國政府報告書,以黃紙為封,不是:黃
Sunday 9 June 2013
INTJ
The N stands for intuition; we actually rely on it a lot with respect to first impressions. For instance, I will probably not even notice what you’re wearing. However, I will remember how seeing you made me feel, what I saw in your eyes, etc.
Wednesday 22 May 2013
出逢った頃のように
My love is forever
Like when we first met
Even when the seasons change
It will never fade
I'm getting tired
Of love manuals and horoscopes
And I'm slowly beginning to get impatient
With everyone around me changing
I mark the days we can meet in my diary
It's kind of strange
I'm even crazier about you than before
Is it a summer love magic?
My love is forever
Like when we first met
I want to be with you forever
I treasure this love
Your clear blue eyes
Make my heart beat faster
Even when the seasons change
It will never fade
I wonder how long we talked on the phone
It still wasn't enough, was it?
What should I wear for our date tomorrow?
It's strange how worried I am
You're always close to me
Watching over me
It makes me feel as tender
As the white waves
I embrace
My growing love and joy
Yeah, I can believe
That you'll still be here next year
My love is forever
Like when we first met
I want to be with you forever
I treasure this love
Your clear blue eyes
Make my heart beat faster
Even when the seasons change
It will never fade
Thursday 16 May 2013
Thursday 25 April 2013
Thursday 18 April 2013
PCR-ESI-MS
http://hawaii.gov/health/laboratories/sld-forms/np-2009_AMP_Poster_Leptospirosis_19F.pdf
use broad range PCR primer pairs e.g. for 16S rRNA conserved region
do PCR->generate a mass spectrum->base composition as a signature to determine microbes.
use broad range PCR primer pairs e.g. for 16S rRNA conserved region
do PCR->generate a mass spectrum->base composition as a signature to determine microbes.
Anti-dsDNA Antibody by immunofluorescence
Crithidia luciliae
•a giant mitochondion containing dsDNA (kinetoplast)
•dose not contain any other antigens present in the cell nuclei
•a giant mitochondion containing dsDNA (kinetoplast)
•dose not contain any other antigens present in the cell nuclei
•Indirect immunofluorescence
•Crithidia lucialiae as substrate
•Positive for anti-dsDNA Ab
•Crithidia lucialiae as substrate
•Positive for anti-dsDNA Ab
Biotinidase assay
• Biotinidase cleaves the amide bond of biotinyl-paminobenzoic
acid (B-pABA, an artificial substrate) to
give free biotin and p-aminobenzoic acid (pABA)
• The pABA is diazotized and reacted with a naphthol
reagent to give a purple product which can be
quantitated spectrophotometrically at 546nm
• Biotinidase activity is expressed as nmol of pABA
liberated per minute per ml serum
• Biotinidase deficient cases <0.7 nmol pABA/min/ml
acid (B-pABA, an artificial substrate) to
give free biotin and p-aminobenzoic acid (pABA)
• The pABA is diazotized and reacted with a naphthol
reagent to give a purple product which can be
quantitated spectrophotometrically at 546nm
• Biotinidase activity is expressed as nmol of pABA
liberated per minute per ml serum
• Biotinidase deficient cases <0.7 nmol pABA/min/ml
Sunday 31 March 2013
immobilized metal-affinity chromatography (IMAC)
IMAC is based on the interactions between a transition metal ion (Co2+, Ni2+, Cu2+, Zn2+) immobilized on a matrix and specific amino acid side chains. Histidine is the amino acid that exhibits the strongest interaction with immobilized metal ion matrices, as electron donor groups on the histidine imidazole ring readily form coordination bonds with the immobilized transition metal.
e.g. Polyhistidine-tag column

e.g. Polyhistidine-tag column

Monday 25 March 2013
Jenner's Stain
Jenner's Stain (methylene blue eosinate) is used in microscopy for staining blood smears.
Wright's stain
Wright's stain is a histologic stain that facilitates the differentiation of blood cell types. It is used primarily to stain peripheral blood smears and bone marrow aspirates which are examined under a light microscope. In cytogenetics it is used to stain chromosomes to facilitate diagnosis of syndromes and diseases.
Warthin–Starry stain
The Warthin–Starry stain (WS) is a silver nitrate-based staining method (a silver stain) used in histology. It was first introduced in 1920 by American pathologists Aldred Scott Warthin (1866-1931) and Allen Chronister Starry (1890-1973), for the detection of spirochetes.[1][2] It has been considered the best stain for detection of spirochetes,[3] and is also used to stain Helicobacter pylori, Lawsonia intracellularis,[4] Microsporidia,[5][6] and particulates.[7]
WS stains organisms dark brown to black, and the background light golden brown/golden yellow.
Carbol fuchsin
Carbol fuchsin, carbol-fuchsin, or carbolfuchsin, is a mixture of phenol and basic fuchsin, used in bacterial staining procedures. It is commonly used in the staining of mycobacteria as it has an affinity for the mycolic acids found in their cell walls.
It is a component of Ziehl-Neelsen stain. Carbol fuchsin is used as a dye to detect [acid fast bacteria] because it is more soluble in the cells wall lipids than in the acid alcohol. If the bacteria is acid fast the bacteria will retain the initial red color of the dye because they are able to resist the destaining by acid alcohol.
Carbol-fuchsin is also used as a topical antiseptic.
Dieterle stain
The Dieterle stain is a way of marking tissue for microscopic examination.
It is used to find the organisms that cause cat-scratch disease and syphilis and sensitive for mycobacterium tuberculosis.
Treponema pallidum particle agglutination assay
The treponema pallidum particle agglutination assay (also called TPPA test) is an indirect agglutination assay used for detection and titration of antibodies against the causative agent of syphilis, Treponema pallidum subspecies pallidum.
In the test, gelatin particles are sensitized with T. pallidum antigen. Patient serum is mixed with the reagent containing the sensitized gelatin particles. The particles aggregate to form clumps when the patient serum is positive for syphilis. In other words, the patient's serum contains antibodies to T. pallidum. A negative test shows no clumping of gelatin particles. This is a type of specific treponemal test for syphilis.
Rapid plasma reagin
Rapid Plasma Reagin (RPR) refers to a type of test that looks for non-specific antibodies in the blood of the patient that may indicate that the organism (Treponema pallidum) that causes syphilis is present. The term "reagin" means that this test does not look for antibodies against the actual bacterium, but rather for antibodies against substances released by cells when they are damaged by T. pallidum.
In addition to screening for syphilis, an RPR level (also called a "titer") can be used to track the progress of the disease over time and its response to therapy.
Friday 8 March 2013
Sailor Stars Song
Give sadness now a sailor smile
Bringing a miracle, a sailor wing
Everybody carries a shining star inside
I won't give up! To tomorrow, a sailor yell
For sure! I will catch it! The sailor star
Let this vow sound through the whole galaxy
After the time when you disappeared from me
I began my journey searching for you
The start is on the map, the stenciled picture of an angel
The destination it points to, a waiting dark colisseum
In my trembling heart, the secret kiss of that day
However hard destiny may be
I will keep following it
I won't look back, with my sailor eyes
Reaching out to you, with my sailor wind
This song is the guidepost of the stars
I won't give up! To tomorrow, a sailor yell
For sure! I will find it! The sailor star
With the wings of an angel, I take off
Alone I run along the road of the unknown
At last I have arrived, here at this fort
At the bottom of the flask, which you left behind
A single piece of the star of trial, now chant the magic spell
This is our miraculous destiny
The past and the future, crossing them all, to catch up with you
Give sadness now my sailor eyes
Bringing a miracle, a sailor wing
Everybody carries a star of fate inside
I won't give up! To tomorrow, a sailor yell
For sure! I will catch it! The sailor star
Let this vow sound through the whole galaxy
I won't look back, with my sailor eyes
Reaching out to you, with my sailor wind
This song is the guidepost of the stars
I won't give up! To tomorrow, a sailor yell
For sure! I will find it! The sailor star
With the wings of an angel, I take off
Friday 15 February 2013
Nanosequencing
-based on the electrical characterization of individual nucleobases
A nanopore is simply a small hole, of the order of 1 nanometer in internal diameter. Certain porous transmembrane cellular proteins act as nanopores, and nanopores have also been made by etching a somewhat larger hole (several tens of nanometers) in a piece of silicon, and then gradually filling it in using ion-beam sculpting methods which results in a much smaller diameter hole: the nanopore. Graphene[3] is also being explored as a synthetic substrate for solid-state nanopores.
The theory behind nanopore sequencing is that when a nanopore is immersed in a conducting fluid and a potential (voltage) is applied across it, an electric current due to conduction of ions through the nanopore can be observed. The amount of current is very sensitive to the size and shape of the nanopore. If single nucleotides (bases), strands of DNA or other molecules pass through or near the nanopore, this can create a characteristic change in the magnitude of the current through the nanopore.
Alpha hemolysin (αHL), a nanopore from bacteria that causes lysis of red blood cells. bind an exonuclease onto the αHL pore. The enzyme would periodically cleave single bases, enabling the pore to identify successive bases. Coupling an exonuclease to the biological pore would slow the translocation of the DNA through the pore, and increase the accuracy of data acquisition.
homomeric αHL pore that has a ring of seven ardinines
located near the barrel of the pore. To bring the diameter of
the conducting pathway closer to the size of the DNA a
cyclodextrin adapter is fitted within the pore.
Mycobacterium smegmatis porin A (MspA) is the second biological nanopore currently being investigated for DNA sequencing. A natural MspA, while favorable for DNA sequencing because of shape and diameter, has a negative core that prohibited single stranded DNA(ssDNA) translocation. The natural nanopore was modified to improve translocation by replacing three negatively charged aspartic acids with neutral asparagines.
A nanopore is simply a small hole, of the order of 1 nanometer in internal diameter. Certain porous transmembrane cellular proteins act as nanopores, and nanopores have also been made by etching a somewhat larger hole (several tens of nanometers) in a piece of silicon, and then gradually filling it in using ion-beam sculpting methods which results in a much smaller diameter hole: the nanopore. Graphene[3] is also being explored as a synthetic substrate for solid-state nanopores.
The theory behind nanopore sequencing is that when a nanopore is immersed in a conducting fluid and a potential (voltage) is applied across it, an electric current due to conduction of ions through the nanopore can be observed. The amount of current is very sensitive to the size and shape of the nanopore. If single nucleotides (bases), strands of DNA or other molecules pass through or near the nanopore, this can create a characteristic change in the magnitude of the current through the nanopore.
Alpha hemolysin (αHL), a nanopore from bacteria that causes lysis of red blood cells. bind an exonuclease onto the αHL pore. The enzyme would periodically cleave single bases, enabling the pore to identify successive bases. Coupling an exonuclease to the biological pore would slow the translocation of the DNA through the pore, and increase the accuracy of data acquisition.
homomeric αHL pore that has a ring of seven ardinines
located near the barrel of the pore. To bring the diameter of
the conducting pathway closer to the size of the DNA a
cyclodextrin adapter is fitted within the pore.
Mycobacterium smegmatis porin A (MspA) is the second biological nanopore currently being investigated for DNA sequencing. A natural MspA, while favorable for DNA sequencing because of shape and diameter, has a negative core that prohibited single stranded DNA(ssDNA) translocation. The natural nanopore was modified to improve translocation by replacing three negatively charged aspartic acids with neutral asparagines.
Co-transfection
1. Viral proteins + cDNA --> Virus --> transduction --> produce protein of interest
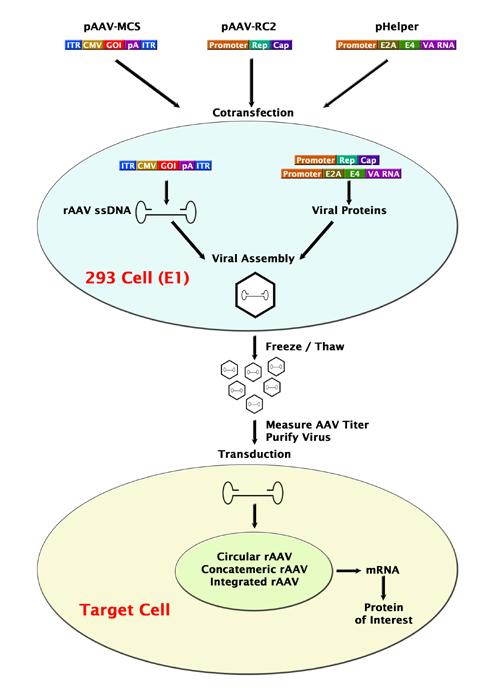
2. Cre/loxP recombination --> gene disturbance / addition of a gene
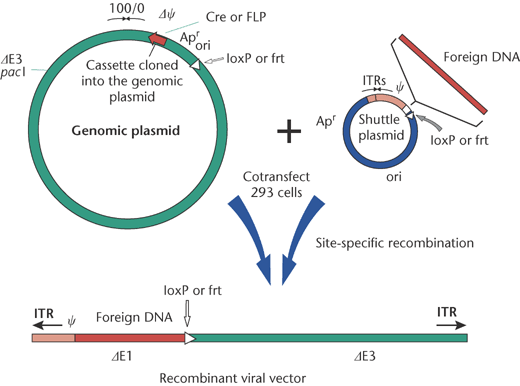

2. Cre/loxP recombination --> gene disturbance / addition of a gene

Tuesday 12 February 2013
MALDI-TOF MS
MALDI-TOF MS:
-consist of 3 components: (i) a specimen ionization chamber, within which the laser-based vaporization of the specimen takes place;
(ii) a time of flight mass analyzer; and
(iii) a particle detector
- sample is spotted onto a target
plate, along with a chemical matrix (5).
The matrix is selected for certain prop-
erties, including easy sublimation, e.g. α-cyano-4-hydroxycinnamic acid
-sample-matrix
mixture is pulsed with a laser, Ultra-
violet nitrogen lasers (337 nm)
- Laser irradi-
ation results in vibrational excitation of
the matrix and the ejection (desorption)
of analyte molecules surrounded by
clusters of matrix molecules, water, and
ions. Once desorbed, the matrix mole-
cules transfer protons to the analyte,
resulting in positively charged analyte
cations in the gas phase.
-accelerated
across an electric field within the ion-
ization chamber to a velocity that
depends on the mass-to-charge (m/z)
ratio of the analyte.
New Role in TB testing
Quantiferon-TB assay (QFT):
Collect blood-->simulate cells with purified protein derivative (PPD)-->detect IFN-gamma by enzyme immunoassay (EIA), which serve as an indirect indicator of exposure to TB
QFT Gold (QFT-G):
-replaced the PPD antigen with early secreoty antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10)
-phytohemagglutinin (PHA) as a +ve control, a mitogen to ensure cells are responding to antigens
-+ve control must show a production of IFN-gamma >=0.5IU/ml
-positive immune response to TB if IFN-gamma >=0.35IU/mi after substracting the value of the Nil control
-IFN-gamma <0.35IU/ml and IFN-gamma of +ve control >=0.5IU/ml ==> unlikely to have TB infection
The manufacturer’s package insert states that a positive QFT result does not “necessarily indicate the presence or absence of active tuberculosis disease.” Other diagnostic procedures, such as mycobacterial culture and radiologic examination, should be used when there is clinical suspicion of TB disease.
Monday 11 February 2013
Sulfonylurea
Sulfonylurea (UK: sulphonylurea) derivatives are a class of antidiabetic drugs that are used in the management of diabetes mellitus type 2. They act by increasing insulin release from the beta cells in the pancreas.
Mechanism of action
Sulfonylureas bind to an ATP-dependent K+(KATP) channel on the cell membrane of pancreatic beta cells. This inhibits a tonic, hyperpolarizing efflux of potassium, thus causing the electric potential over the membrane to become more positive. This depolarization opens voltage-gated Ca2+ channels. The rise in intracellular calcium leads to increased fusion of insulin granulae with the cell membrane, and therefore increased secretion of (pro)insulin.
Subscribe to:
Posts (Atom)
